Pursuing major unmet
needs with our VLP technology
A robust pipeline targeting multiple respiratory diseases
We are advancing VLP vaccine candidates that have the potential to induce high neutralizing antibody (NAb) titers for more effective protection. These vaccines are intended to help protect the most vulnerable populations, including older adults, against severe disease.
Progressing multiple assets:

*FDA Fast Track designation.
†Icosavax does not plan to pursue the IVX-121 RSV monovalent candidate as a standalone candidate for RSV in older adults, and has transitioned development to the IVX-A12 bivalent RSV/hMPV candidate following Phase 1/1b.
‡Icosavax has worldwide nonexclusive rights with exception of South Korea (which is not included in the licensed territory), which will convert to exclusive rights in North America and Europe (including Switzerland and United Kingdom) starting in 2025, with non-exclusivity maintained elsewhere.
Respiratory vaccines are an emerging market. There is unmet need for an RSV and hMPV combination, and our lead bivalent vaccine candidate, IVX-A12, targets these two common causes of pneumonia in older adults. Better protection from flu is also needed and preclinical candidate development is in progress.
Top 5 pathogens detected in adults hospitalized with community-acquired pneumonia (EPIC study; pre-COVID-19)1

Our first combination vaccine candidate, IVX-A12, targets BOTH RSV and hMPV, two of the top five causes of pneumonia in adults1

Icosavax is currently conducting a Phase 2 trial for IVX-A12 for the prevention of RSV and hMPV. This follows the reporting of positive topline interim Phase 1 data§ in May of 2023.
IVX-A12 is a potential first-in-class combination vaccine candidate containing VLPs that incorporate stabilized prefusion F proteins from RSV and hMPV viruses. The FDA has granted IVX-A12 Fast Track designation in adults ≥60 years of age. We are the only company pursuing RSV and hMPV with the VLP modality, as well as the only company with a combination RSV/hMPV candidate in the clinic for older adults.
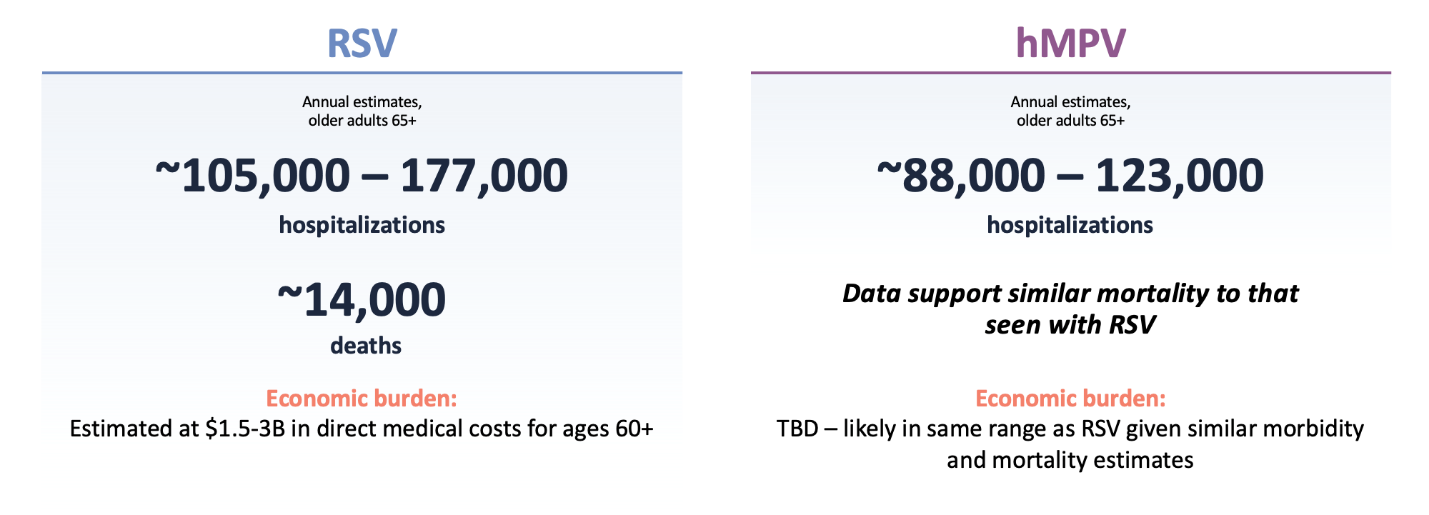
RSV and hMPV have a significant impact on the older adult population
RSV and hMPV are related Pneumoviridae that have overlapping seasonal circulation. Both viruses are common, with high re-infection rates. A vaccine able to prevent both RSV and hMPV has the potential to almost double the health and economic impact of a vaccine targeting RSV alone, assuming similar efficacy against both pathogens.
RSV and hMPV: US health and economic burden (older adults)2-5
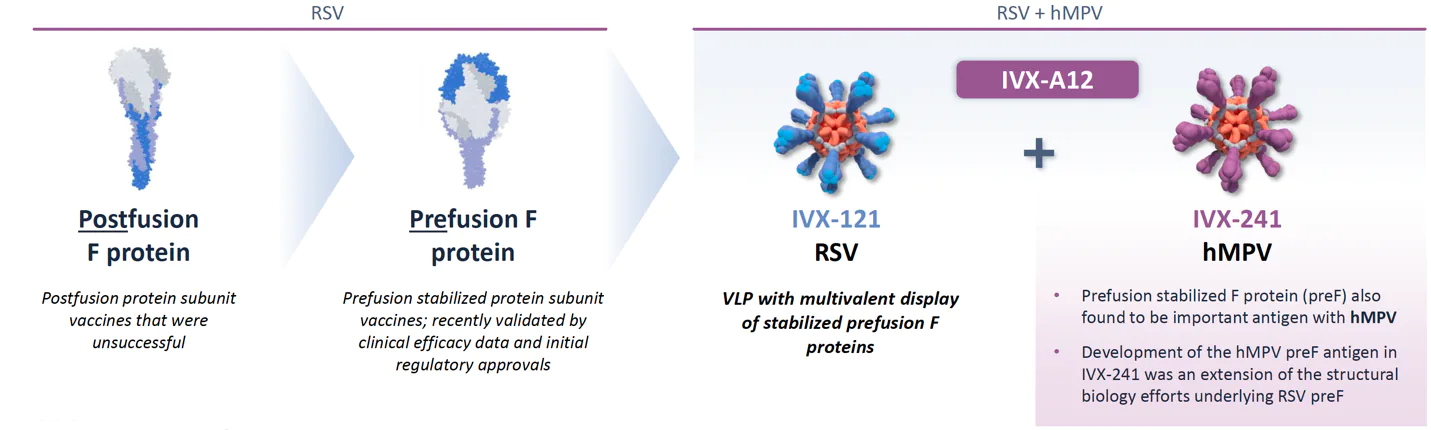
Leveraging a breakthrough innovation in the field, we are utilizing prefusion stabilized F antigens for display on our VLPs for both RSV and hMPV
In our RSV/hMPV development program, we have focused on developing and advancing VLPs that stimulate high NAb titers. Every log improvement in NAb titers is believed to lead to a clinically relevant improvement in disease outcome.
Both our RSV and hMPV VLPs employ the F protein responsible for viral cell entry. F proteins undergo conformational changes upon fusing to the cell membrane, and utilizing prefusion F proteins may lead to higher NAb titers.
The transition to prefusion F protein was a game changer in RSV vaccine development, and IVX-A12 incorporates stabilized prefusion F proteins for both RSV and hMPV
Clinical development is progressing following positive topline results with IVX-121 (the RSV portion of IVX-A12) and IVX-A12
Positive IVX-121 (RSV VLP) Phase 1/1b and Phase 1b extension immunogenicity data provide an initial indication of potential differentiation of the VLP technology on durability and revaccination6
Briefly, data show:
- High RSV-A and RSV-B neutralizing antibody titers seen from the lowest dose tested
- Similarly robust responses in older vs. young adults
- Favorable tolerability
- Suitability for combination (tolerability profile up to maximum dose tested in Phase 1 [250 µg] and immunogenicity down to 25 µg gives room for multivalent combinations)
- Six-month immunogenicity update showed durability of RSV-A and RSV-B neutralizing antibody titers up to 180 days after vaccination, with geometric mean titers (GMT) against RSV-A through Day 180 persisting at 64%–98% of the GMTs at Day 28 in older adults
- Positive IVX-121 Phase 1b 12-month immunogenicity data in older adults, and initial evidence for revaccination potential with VLP-based vaccine
- GMTs against RSV through Day 365 persisted at ~45%–70% of the GMTs at Day 28 (for 75 and 250 µg unadjuvanted dosages)
- Data provide additional clinical evidence of potential differentiation on durability with company’s VLP platform technology
- Robust immune response against RSV-A observed in Phase 1b extension trial participants who were revaccinated with IVX-121 approximately twelve months following initial dose
- IVX-121 continues to be generally well tolerated with no additional safety concerns observed with longer-term follow up or revaccination
Positive IVX-A12 (RSV + hMPV VLPs) Phase 1 topline interim results§ showed robust immune responses to both RSV and hMPV in older adult subjects6
This was the first demonstration of hMPV vaccine immunogenicity in an older adult population for the field. Briefly, these data show:
- Higher post-vaccination levels of RSV-A and RSV-B antibody titers (NAbs) (IU/mL) in this IVX-A12 study than historically seen with IVX-121 (RSV) alone
- No evidence of immune interference between RSV and hMPV and a similar pattern of response
- Tolerability up to highest dose tested, allowing for a full range of formulations in Phase 2
- Systemic adverse events comparable to placebo; mild reactogenicity across formulations tested
- Prespecified sub-analysis showed particularly strong responses in those with the lowest baseline titers
These data support progression to a Phase 2 safety and immunogenicity trial in a larger older adult population. Data from the Phase 2 study will help us to select the final formulation for a subsequent Phase 2b proof-of-concept disease prevention study.
IVX-A12 Phase 1 and 2 trials are designed to inform bivalent formulation prior to anticipated proof-of-concept disease prevention efficacy study
- Safety and immunogenicity of bivalent (RSV/hMPV) formulations
- Healthy older adults (N = 140)
- Constant RSV dosage (75 µg) + multiple hMPV dosage levels (75, 150, 225 µg)
- Single dose +/- adjuvant (MF59®)
- Duration follow-up planned to one year
- Safety and immunogenicity; dosage and formulation selection
- Healthy older adults with wider age range and comorbidities (N ≈ 250)
- Two RSV and hMPV bivalent formulations (150 µg RSV VLP + 150 µg hMPV VLP +/- adjuvant)||
- Planning for long-term safety and duration of immunogenicity (multi-year) from selected formulation(s)
- Safety, immunogenicity and protection against hMPV infectious challenge
- Healthy adults
- Single formulation based on Phase 2 interim data
- hMPV challenge model development in progress
Assess safety and immunologic non-interference with varying ratios of RSV/hMPV VLPs
Allows assessment of immune responses to hMPV and RSV with and without adjuvant
Conduct further dose optimization with expanded safety and immunogenicity evaluation in older adults; assess longer-term safety and durability of immune response
Assessment of efficacy potential in hMPV build understanding of symptom profile to inform Phase 3
Flu is an important part of our vision
A VLP-based vaccine targeting influenza—potentially deployed as part of a combination vaccine—is being pursued. In relation to this initiative, we also have plans to build a rapid production system capable of supporting seasonal flu, which we expect to also enhance our pandemic response capabilities. Preclinical candidate development is in process.
There is an unmet need for a flu vaccine with improved efficacy, particularly in the older adult population. Even though numerous marketed vaccines are available, flu still causes ~380K hospitalizations and ~20K deaths/year in the US—the majority in people over 65.7 In addition, despite their commercial success, existing flu vaccines have historically had suboptimal efficacy (~20%–50% over the last 10 years) and need to be updated every season.7
Better vaccines for COVID-19 are needed
We believe there will be a need for better vaccines and that a meaningful opportunity could persist beyond the pandemic in COVID-19, and this is an area of interest to the company for its optionality as a potential component of our combination vaccines vision.
Preclinical COVID-19 candidate development is in process, and we are currently focusing on a bivalent strategy for a COVID-19 candidate displaying computationally engineered receptor binding domain (RBD) antigens. The objective is antigen prototypes that improve the stability of the RBD subdomain from the SARS-CoV-2 S protein and could allow for the successful presentation of evolving variants of interest, including omicron and subsequent derivatives.
1. CDC, EPIC study. 2. Sieling WD et al. Influenza Other Respiratory Viruses. 2021;15(5):670-677. 3. Widmer K et al. J Inf Dis. 2012; 206(1):56-62. 4. Falsey AR et al. NEJM. 2005;352(17):1749-1759. 5. Herring WL et al. Vaccine. 2022;40(3):483-493. 6. Data on file. Icosavax, Inc. 7. CDC, flu seasonal burden and seasonal flu vaccine effectiveness studies.
Icosavax is committed to the development of vaccines designed to address significant unmet public health and medical needs. Our goal is to ensure access to our investigational vaccines at the appropriate time and in a clinically appropriate manner as determined by your physician.
At this time, Icosavax believes that participation in one of our clinical trials, which are carefully designed to determine the safety and immunogenicity or efficacy of an investigational vaccine, is the best and most appropriate way to access our investigational vaccines. We do not currently provide our investigational vaccines for use through expanded access.
If you are interested in accessing any of our investigational vaccines, please speak with your physician. You may also learn more about ongoing clinical trials by going to clinicaltrials.gov and searching for Icosavax.
Consistent with the 21st Century Cures Act, Icosavax may revise this policy at any time.
