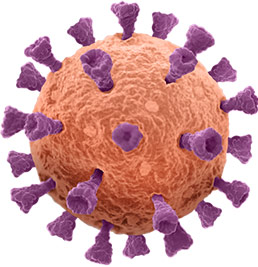
Mimicking the structure of viruses to empower a better immune response
Taking a proven approach to the next level
At the center of our novel approach to vaccines is breakthrough virus-like particle (VLP) technology. Born out of a collaboration between the Gates Foundation and the University of Washington’s Institute for Protein Design, this technology enables high-density, multivalent display of antigens in a manner that closely resembles the structure of a virus. This is believed to induce a stronger and more durable immune response vs. traditional soluble antigens. Initial validation for our approach has come via positive Phase 1/1b topline data for our VLP-based vaccine candidate targeting RSV and positive Phase 1 topline data for our bivalent candidate targeting RSV and hMPV.
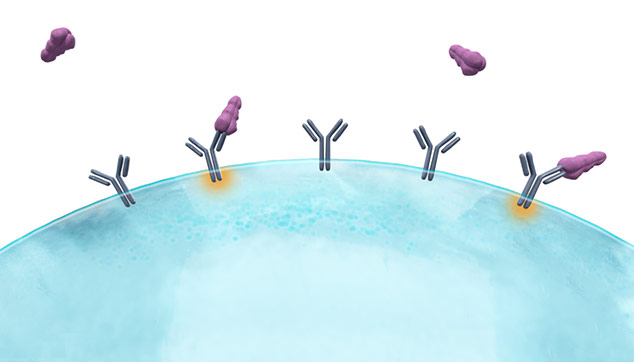
Unlike soluble antigens, VLPs mimic the structure of real viruses
Natural virus

Soluble antigen
Traditionally manufactured or mRNA-derived

VLP-based antigen
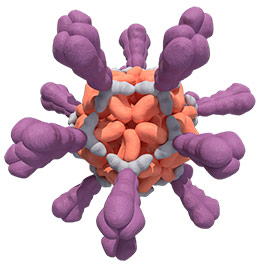
Because the body “sees” VLPs as viruses,
the result may be a stronger, more durable immune response
(traditionally manufactured or mRNA-derived)
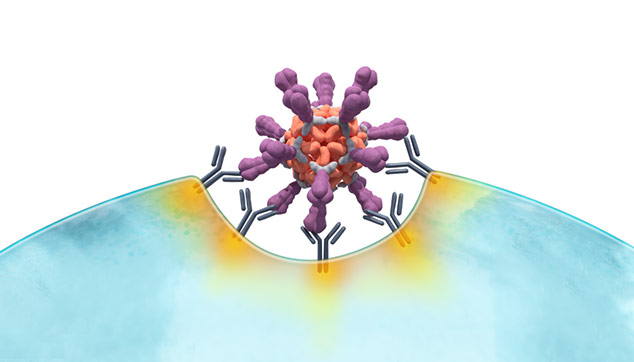
Adapted from B. Graham, NIH, ResViNet 2017 presentation.
Multiple mechanisms may underpin the anticipated robust immune response to VLPs

Adapted from Gomes AC, Mohsen M, Bachmann MF. Vaccines. 2017;5(1):6.
How VLPs are made
VLPs are a proven technology with multiple products on the market, including vaccines for HPV and HBV. While currently available vaccines utilize the few proteins that naturally fold into VLPs, we’re building on that success with intentionally designed VLPs.
Our VLPs are produced via a proprietary, 2-component, computationally-
designed system
Potential benefits of VLPs
When compared to existing modalities, we believe our VLP technology has the potential to improve upon:
Magnitude of response
To counter immunosenescence that can occur in older adults
Breadth of coverage
Greater degree of protection against related viral strains and mutations; less customization for variants
Durability
Longer antibody persistence and requiring fewer boosters
Tolerability/reactogenicity
Lower incidence of side effects and greater acceptability
Manufacturing
High productivity and scalability with process efficiencies, storage flexibility, and stability
Combinability
Ability to combine multiple VLPs into one vaccine
